Management of hypertension is one of the most important tasks of nephrologists, which if done well can be highly rewarding. More often than not, blood pressure is either left uncontrolled or is lowered too much, putting patients at risk of the attendant complications. Realising the fallacy and dangers of ‘one size fits all’ approach which has been the part of preexisting guidelines, ACC/AHA 2019 guidelines, were a much needed departure from the ‘target based’ to the ‘risk based’ approach of BP treatment. This is a sensible approach, when one views BP elevation as a risk factor for organ damage/dysfunction and not a disease in itself, with lesser emphasis on moving a BP number from a higher to lower value and greater emphasis on reducing the risk posed by elevated BP to the health.
However, KDIGO 2021 HTN guidelines seem to be traveling back in time to embrace the target blood pressure, the magical number <120 systolic for the entire CKD population (except those on dialysis).
This recommendation not only lacks sufficient evidence, but if implemented strictly by treating doctors, can be potentially disastrous. A recent review has summarised the serious limitations of the evidence on which KDIGO 2021 BP target is based, and we will discuss here the basis for more conservative BP targets for most patients with CKD.
Why is BP elevated in patients with CKD?
Blood Pressure=Cardiac Output x Systemic Vascular Resistance
Given the fact that most patients with CKD will have high BP, what factors elevate CO or SVR in these patients?
Disturbance of sodium and volume regulation (not appreciable as edema in early CKD), activation of RAS and sympathetic nervous system, anemia and multiple other factors determine BP by affecting CO or SVR. This makes hypertension in CKD pathophysiologically complex and different from primary hypertension(figure 1). Therefore, the data guiding treatment of primary hypertension can’t be directly extrapolated to HTN in CKD. Hypertension is not a disease in itself but a risk factor for organ damage/dysfunction and consequent morbidity and mortality. Risk with hypertension in CKD is two folds: cardiac plus renal. One also needs to acknowledge the nature of CV disease in CKD which isn’t all the same as the general population without kidney disease. Uncontrolled BP is one of the most important determinants of CKD progression along with proteinuria, and multiple pivotal trials in diabetic CKD –Captopril trial, RENAAL, IDNT-(although they were not BP target trials) have shown the efficacy of antihypertensive therapy in renoprotection.
Figure 1. Pathogenesis of hypertension in chronic kidney disease
Limited generalisability of SPRINT CKD to the real world CKD
Data supporting BP target of <120 systolic comes from SPRINT trial.
First of all, one needs to understand that SPRINT-CKD, unlike EMPA-KIDNEY or DAPA CKD, isn’t an independent randomised controlled trial, and is a pre-specified subgroup analysis of SPRINT trial.
If you wish to prepare a checklist of ‘refer to nephrology’ for a primary care doctors clinic, you may rather copy paste SPRINT trial exclusion criteria which go like this: diabetes, proteinuria >1gm/day, ADPKD, glomerulonephritis, eGFR <20 (in addition to age <50). Mean serum creatinine in this elderly population (age 67 yrs), was 1.56mg/dl, and urinary albumin excretion was ~40 mg/gm. This clearly is not a ‘nephrologist’s patient population’, unless the physician is a very good friend of yours or is too scared by serum creatinine of 1.56mg/dl in an elderly non diabetic. Indeed, the rate of eGFR decline in SPRINT trial is similar to the age associated decline in kidney function. Extrapolating findings from SPRINT CKD cohort to CKD clinics is going to be a mistake that can cost (mostly borne by kidney).
The conclusion of the SPRINT CKD goes like this:
“among patients with CKD and hypertension without diabetes, targeting an SBP<120 mm Hg compared with <140 mm Hg reduced rates of major cardiovascular events and all-cause death without evidence of effect modifications by CKD or deleterious effect on the main kidney outcome” . Even authors themselves (and rightly so) don’t claim to have established a new BP target for CKD. These findings only mean that main SPRINT results are also applicable to the ‘accidental CKD’ like the rest of the population.
Accidental CKD is detected when an elderly patient with comorbidities gets to know that he has CKD after reading the eGFR value in the bold type which at the bottom of the paper also gives the latest KDIGO CKD classification. Telling them “nothing to worry about” and seeing the wrinkles disappear from their faces is one of the happy moments in our practice. This age associated decrease in GFR without significant albuminuria is of questionable significance and is arguably the medicalisation of aging.
Limited generalisability of the SPRINT CKD was highlighted by a study performed across 40 Italian nephrology clinics, involving 2847 patients. This population differed significantly from the SPRINT CKD, specifically, they have higher risk of ESRD, CV deaths and all cause mortality.
Intensive BP lowering may be harmful
An important concern for the broader application of <120 is the harm, and this is particularly relevant to the CKD. Two trials that dealt with a very similar BP target in diabetics and non diabetics (ACCORD BP and SPRINT) reached discordant primary outcomes, however, they concur upon the harm of intensive BP lowering.
In the ACCORD BP trial, serious adverse events attributed to BP lowering were more likely in the intensive lowering arm: hypotension, syncope, bradycardia/arrhythmias. Importantly, in a population at very low risk of progressive CKD (mean creat 0.9mg, median UACR 14mg/gm), risk of of eGFR <30ml (as an adverse lab measure) was doubled with intensive BP lowering [99 (4.2%) vs 52 (2.2%) <0.001]. Alarmingly, renal failure occurred in 5 patients in the intensive control arm versus 1 patient in the less intensive arm.
In SPRINT trial as well, in addition to increased risk of hypotension, syncope and electrolyte abnormalities, intensive lowering led to higher incidence of AKI [204 (4.4%) vs 120 (2.65) HR 1.71 P<0.001], ≥30% reduction in estimated GFR to <60 ml [ 127 (3.85) vs 37 (1.1%) HR 3.49 P<0.001].
A post hoc analysis of SPRINT showed a higher risk of AKI and eGFR based renal outcomes (≥30% decrease in eGFR to a value of <60 mL for participants without CKD; and a composite of ≥50% decrease in eGFR the development of ESRD with CKD) in the intensive control group. This analysis also showed that the CV protective effect of intensive BP lowering is significantly blunted at reduced GFR and was no longer significant at eGFR<45 ml per min(figure 2,3,4).
Figure 2. Risk of AKI is higher in patients with eGFR <45 ml/min( J Intern Med 2018 Mar 283(3):314-327)
Figure 3. eGFR based renal outcomes (≥30% decrease in eGFR to a value of <60 mL for participants without CKD; and a composite of ≥50% decrease in eGFR the development of ESRD with CKD) is higher in intensive BP control group (J Intern Med 2018 Mar 283(3):314-327)
Figure 4. CV protective effect of intensive BP lowering is blunted at reduced GFR and was no longer significant at eGFR<45 ml per min (J Intern Med 2018 Mar 283(3):314-327)
Many patients in nephrology clinics have severe hypertension, often symptomatic with headache, visual disturbances (This is another dark room in nephrology awaiting to be lit). These patients are younger and don’t have other traditional CV risk factors like dyslipidemia or atherosclerotic CVD. How will this group fare with intensive BP lowering? An interesting analysis using patient-level data from 9361 randomised participants of SPRINT trial evaluated a similar issue. They studied the impact of baseline BP and Framingham risk score on outcomes, and showed that in participants with a baseline SBP of at least 160 mmHg and a lower Framingham risk score, targeting an SBP of less than 120mmHg compared with less than 140 mmHg resulted in a significantly higher rate of all-cause death [hazard ratio (95% CI) for intensive group: 3.12 (1.00-9.69); P = 0.049].
SPRINT trial was terminated early after 3.5 years, and therefore the long term impact of these renal events on CKD outcomes remains a question and there is no reason to believe in long term safety of intensive BP control. Premature termination of RCTs carries the risk of overestimating the benefits and underestimating the harm of the intervention. What will happen to this renal function decline over years of follow up? The correct answer to this question is “we don’t know”. However, KDIGO prefers to dismiss it as a hemodynamic and transient phenomenon. Although ‘nephrotoxicity’ of intensive BP lowering hasn’t bothered trialists and KDIGO guideline makers, the risk benefit of intensive BP lowering needs to be carefully discussed with our patients before implementation.
Why was there a dissociation between CV and renal benefits in SPRINT?
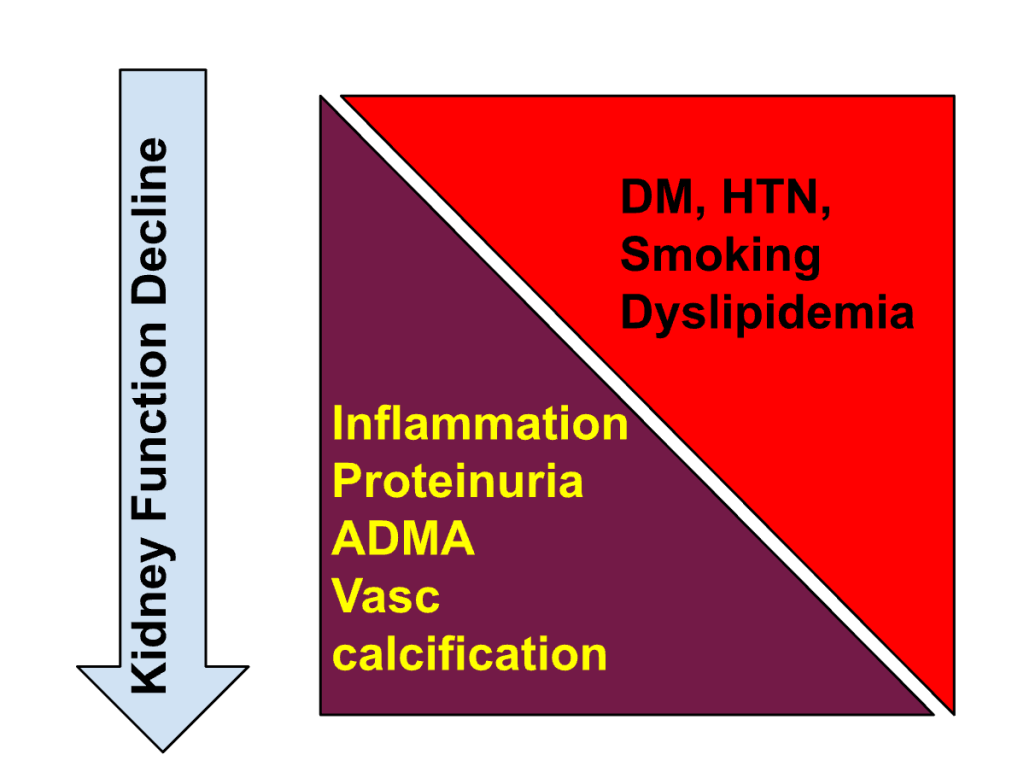
Figure 5. Spectrum of risk factors for CV events changes with progression of CKD
Is ‘some renal compromise’ an inevitable consequence if you wish to gain CV benefits? We believe that this apparent ‘cardiorenal paradox’ has to do with the different nature of CV risk profile in CKD. With worsening kidney function, the nature of CV risk changes, with greater contribution from non-traditional CKD specific risk factors (in addition to traditional atherosclerotic CV risk factors)(fig 5). CKD in a nephrologist’s clinic resembles more to the participants in the landmark CKD-BP trials: Captopril study, REIN , RENAAL and IDNT, MDRD and AASK. Unlike SPRINT or SPRINT-CKD, most of these trials dealt with participants having high risk of CKD progression. In such patients, renoprotection is likely to go hand in hand with cardioprotection, and intervention in addition to kidney disease is likely to offer cardioprotection as well (considering the fact that CV disease in this population is often non-coronary i.e. left ventricular hypertrophy, systolic and diastolic dysfunction, and cerebrovascular disease).
Is KDIGO recommendation valid if standardised BP measurement is universally adopted?
One argument put forth in support of intensive BP lowering is that if we measure BP properly (standardised office BP or AOBP) and minimize white coat effect, a target of <120mmHg will make sense.
We argue that even if one imagines a theoretical scenario where AOBP will be used universally for monitoring BP, intensive BP lowering will still have all the attendant risks that we discussed. In a systematic review and meta analysis, involving 9279 participants, AOBP readings were 7-14 mmHg lower (the difference in the routine office BP and “research settings” was ~7 mmHg and that between routine office and AOBP was 14 mmHg). Even if we adjust for the way BP is recorded, none of the pivotal CKD trials (even post SPRINT) had reached target SBP <120 mmHg(table 1).
| Clinical trial | Blood pressure achieved (intervention vs control) |
| Captopril | 128-134 vs 129-136 |
| IDNT | 141/77 vs 144/80 |
| RENAAL | 140/74 vs 142/74 |
| CLICK | 130/70 vs 140/75 |
| DAPA CKD | ??? vs 137/78 |
| EMPA KIDNEY | control arm BP 137/78 (Reduction in SBP was−2.6 mm Hg in intervention) |
Table 1. List of randomised control trials on CKD patients. with serum creatinine values at the enrolment and target BP achieved in the trial. As can be seen, even in the research settings the target BP of 120 mmHg was not achieved in landmark trials involving patients with CKD
What do we already know about BP targets in CKD?
Before SPRINT-CKD, what did we know about intensive BP lowering in non diabetic CKD? Two trials that evaluated the effect of intensive versus less intensive BP targets in real world non-diabetic CKD are MDRD (serum creatinine 2-3 mg/dl) and AASK (serum creatinine 2-2.2mg/dl, >30% participants had UACR >0.22). Both assessed impact of this intervention primarily on kidney function, as decline in eGFR, and concluded that lower target (<125 in MDRD, <129 in AASK) doesn’t offer additional renoprotection than usual targets. Subgroup analysis of patients with proteinuria suggested the benefit of lower target on kidney outcomes. Post trial follow up of AASK and MDRD (for 14-15 years) claimed reduced overall mortality (HR 0.87, 95% CI 0.76-0.90), and also reduced the progression to ESRD (HR 0.88, 95% CI 0.78-1.00), but the benefit was confined to those with proteinuric CKD. These findings from post trial follow up should be interpreted with caution: they are discordant with primary outcome of the original trials, observed differences in outcomes are marginal, and importantly, there was no difference in the blood pressure between the groups in the follow up period. Several meta-analyses combining these trials of various designs, interventions, different BP targets, not surprisingly, have reached different conclusions claiming (here and here) and refuting (here and here) the benefit of intensive BP lowering.
Nephrology dark room
Figure 6. There is a lack of data about BP targets in advanced CKD, X axis depicting serum creatinine concentration of the trial population
Even the landmark BP-CKD trials have involved the patients with mild to moderate decrease in kidney function and evidence guiding BP treatment in more severe CKD (and not on dialysis) is virtually nonexistent (figure 6). Unfortunately, KDIGO doesn’t make any distinction about the advanced CKD (which may be one of the most prevalent patient populations in nephrology OPDs), implying that target <120 is valid across the spectrum of CKD.
What should clinicians do in the absence of convincing evidence for BP goals in their patients? Patient’s values and preferences should be considered while developing guidelines, KDIGO committee didn’t have patient representation. What would patients with CKD value? Patients and clinicians may believe that intensive BP lowering reduces all cause mortality significantly (relative risk reduction of 28%), however they need to be informed about a much smaller absolute risk reduction of 1.9%. On the basis of SPRINT results, for every 1000 patients treated for 3.2 years with intensive BP lowering compared to the less than 140 systolic, on average, 19 persons will benefit, 26 will experience acute renal failure (ARF),*955 will not experience benefit or harm. Such clear discussion of the risk-benefit should be done with all those patients in whom intensive BP lowering is considered. In our CKD clinic, after screening 119 patients, 12 met inclusion criteria, of which only 2 agreed to go for the intervention of intensive BP lowering (unpublished data, Tukaram Jamale).
*ARF was the terminology by SPRINT trial in adverse event reporting. It’s interesting to know how this trial defined acute kidney injury. ARF was included as an event if the diagnosis was listed in the hospital discharge summary, and considered by the SPRINT Safety Officer, after reviewing medical records to be one of the top three causes of the admission or continued hospitalization. A few patients with ARF were noted in the emergency department records instead of hospitalization records.
In the opinion of the KDIGO committee, if there is uncertainty about the risk/benefit, it seems ok to consider the benefit and ignore risk. See a paragraph from the guideline document below:
Is there a plausible mechanism of harm?
In patients with long standing hypertension and especially those with comorbidities like CKD, the pressure-flow curve of the blood flow in various organs (e.g. brain) is shifted to the right(figure 7).
Figure 7. In hypertensive patients, the autoregulation curve is shifted to the right.
In other words, the blood pressure threshold that leads to compromised tissue perfusion in hypertensive patients is different (higher) than the general population. Importance of this phenomenon was highlighted in several studies involving patients with chronic hypertension seen in acute care settings. For example, SEPSISPAM trial, that evaluated two different MAP targets in patients with sepsis (80-85 vs 65-70 mmHg), primary outcome of 28 day mortality didn’t differ in the two groups, however, among patients with chronic hypertension, those in the high-target group were less likely to experience the doubling of serum creatinine [90/173 (52.0%) vs 65/167 (38.9%) p=0.02] and required less renal-replacement therapy [73/173 (42.2%) 53/167 (31.7%) p=0.046] than those in the low-target group. Another trial involving peri operative patients evaluated individualized versus standard BP management strategy (aimed at achieving a SBP within 10% of the patient’s resting SBP vs treating when it falls below 80 systolic), individualized management was associated with a non significantly reduced risk of AKI and CNS dysfunction post op, although primary outcome of SIRS and organ dysfunction wasn’t different in these groups. In another study evaluating the effect of higher target BP in patients with prior hypertension, sublingual microcirculation (assessed by sidestream dark field imaging) significantly improved in patients with higher MAP. Given these observations, and the existing data from large RCTs, normalizing (rather than controlling) blood pressure in patients with chronic hypertension is not only unnecessary but can be harmful and the brunt of this harm is borne by the kidney.
Mean Perfusion Pressure (MPP) is given by the difference between Mean Arterial Pressure (MAP) and Central Venous Pressure (CVP) i.e. MPP=MAP-CVP. Another factor that can further compromise MPP in patients with CKD is increased CVP as a result of volume overload, diastolic dysfunction, and increased left and right ventricular filling pressures. This factor is likely to become more prominent with progressive GFR decline. Although KDIGO 2021 BP targets don’t apply to patients on dialysis, in this population the safe targets are likely to be further higher as highlighted by this pilot RCT.
Is the quality of evidence and strength of recommendation 2B or 2C?
KDIGO has graded the evidence supporting intensive BP lowering in CKD as 2B-a suggestion based on moderate quality evidence. We evaluated the evidence in the framework of GRADE, and reached a conclusion that evidence supporting this target is 2B at the best, a suggestion based upon poor quality evidence. Several features of the trials make the case for this:
1. Evidence supporting this is indirect (subgroup analysis and not an independent trial, now, someone may argue that it’s largest in terms of number, but it also is farthest from true CKD in terms of patient characteristics!)
2. There is risk of bias as trials considered are open label
3. Inconsistency of the observations -previous large trial, ACCORD BP, has refuted the hypothesis that intensive BP lowering helps
After having discussed potential risks of the intensive BP lowering, and lack of adequate evidence, and evidence of harm in patients with diabetes, strangely, guidelines have resorted to follow the intensive BP lowering. They applaud SPRINT’s plan of including CKD (GFR 20-59ML); however forget the fact that they ultimately ended up with a participant group with eGFR >70ml ( serum creatinine ~1 mg/dl). Unlike AASK and MDRD trials who also were set out to include patients with CKD and actually included them, SPRINT couldn’t enroll a real world CKD population. The reasons for selective inclusion of patients with higher GFR in SPRINT are unclear but one of them may be the perceived risk of intensive lowering in patients with lower GFR by the site physicians.
Summary
In the vast majority of the CKD patients in nephrology OPDs, evidence to lower BP target of <120 is nonexistent, and therefore guideline recommendation of <120 is not supported by the good evidence. With progressive decrease in kidney function, you get to the nephrology dark room where no evidence to guide BP management exists. Nephrologists, therefore shoulder the responsibility of treating one of the most difficult blood pressure challenges with no or little evidence to base their decision upon.
After careful evaluation of the available evidence, one would rather choose to maintain the equipoise about BP goal and individualize it for different situations after shared decision making with the patients.
